
Balázs Győrffy and project members
The research focuses on tumours of the central nervous system, more precisely medulloblastoma, the most frequent type of paediatric malignant brain tumours. Balázs Győrffy, scientific advisor of the 2nd Dept. of Paediatrics of the Semmelweis University, head of a Momentum research group, awarded with the Officer’s Cross of the Order of Merit of Hungary (civilian) this year, shared with us information on the special therapeutic approach, which can even be tailored to specific, individual needs.
We already know that tumours do not originate in the fully developed neural cell – Balázs Győrffy explains – medulloblastoma develops directly from the progenitor of these cells. In addition, there are certain tumours of the central nervous system that develop from the progenitors of glial cells (glial cells surround, support and protect neurons).
According to the protocol to be followed after the disease has been diagnosed, the tumour is surgically removed first, with the least possible impact on intact tissues. This is followed by radiation treatment, which eliminates tumour cells that might have potentially remained. The third step is chemotherapy, pharmacotherapy. Medicines used today were introduced on an ongoing basis as of the 1970s until the 1990s, and though they significantly contribute to a stronger chance of recovery, after more than two decades new medicines would be needed.
Generally, in the process of pharmacological development, a genetic mutation leading to a tumour is the subject of a targeted therapy. For example, the epidermal growth factor receptor (EGFR) contains the genetic error and also needs to be targeted. The approach, which is unprecedented worldwide, taken by Balázs Győrffy et alia applies on a computer algorithm to identify further genes. They also identify the “source” genetic defect, but deliberately do not target the defect gene, but genes whose functioning might be affected by this altered gene. The altered functioning of such genes may also be responsible for the development of the tumour. Consequently, the pharmacological treatment must have multiple targets at the same time.
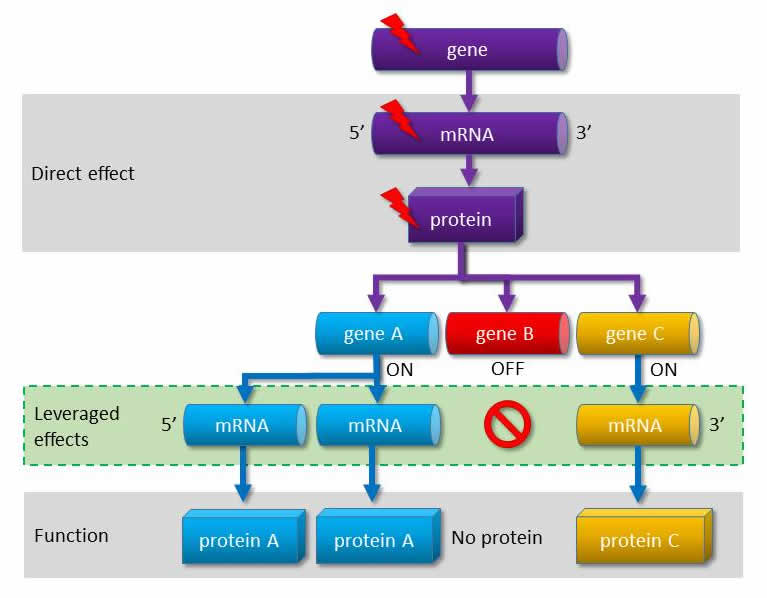 |
| The gene malfunctioning due to the mutation influences the functions of other genes. According to the new approach the pharmacological treatment must have indirect targets. |
First, all the available data need to be collated for the algorithm to function. Patients with tumours are categorised according to whether a genetic malfunction has been identified in them or not, and what their prognosis was like. Then, the algorithm identifies the gene or genes that show the closest correlation with the outcome of the disease in a given group of patients. The aim is to block the functioning of this gene (or genes).
The computer-based work is done at the Semmelweis University. This is followed by the next section of the project, implemented by a group at the Research Centre for Natural Sciences of the MTA, who prepare a substance inhibiting the protein of the gene selected. Another group at the same research centre will then check and analyse the effect of this molecule in a cell line model. The process is continued at the Semmelweis University again: under laboratory conditions cell culture and animal testing are used to confirm whether the prepared molecule does indeed affect the tumour.
- Identifying the error
- Preparing the inhibitor
- Confirming the effect of the inhibitor on the genetic error
- Confirming that the error blocked by the inhibitor contributed to the development of the tumour
Cornerstones in pharmacological engineering
The third project partner is Meditop Gyógyszeripari Kft., specialised in oral drugs (as opposed to all the traditional chemotherapeutical drugs, hardly any of which can be administered orally). This is where the inhibitor, or hopefully several inhibitors may be manufactured, in purity and stability making it possible for them to be administered even to paediatric oncology patients. However, application is preceded by a long process of testing.
Development itself takes a long time: researchers plan to define in the coming one and a half years the genetic pattern of the tumours of children who have been treated with tumours of the central nervous system over the past ten years. Approximately twenty people work on the project under the leadership of four lead researchers, but university students, PhD students and postdoctoral fellows are also involved in the large-volume work.






